Ironwood Pharmaceuticals, Inc., (NASDAQ: IRWD) an entrepreneurial
pharmaceutical company, discovers, develops, and commercializes human
medicines.
Its lead product candidate, linaclotide, is a guanylate
cyclase type-C agonist that completed Phase III clinical trials for the
treatment of patients with irritable bowel syndrome with constipation (IBS-C)
and chronic constipation (CC).
Ironwood Pharmaceuticals (NASDAQ: IRWD) has collaboration
and license agreements with Forest Laboratories, Inc. to co-develop and
co-market linaclotide in North America; Almirall, S.A to develop and
commercialize linaclotide for the treatment of IBS-C, CC, and other lower
gastrointestinal conditions; and Astellas Pharma Inc. to develop and
commercialize linaclotide for the treatment of IBS-C, CC, and other
gastrointestinal conditions in Japan, South Korea, Taiwan, Thailand, the
Philippines, and Indonesia.
More about
Linaclotide:
Linaclotide is a GC-C agonist in development for the treatment
of irritable bowel syndrome with constipation (IBS-C) and chronic constipation
(CC).
Complications of chronic
constipation include haemorrhoids, faecal impaction (shown here on CT scan) and
intestinal perforation | Photo: SPL
The clinical efficacy
portion of the linaclotide development program is complete and two
long-term safety studies are still ongoing.
Across four Phase 3
efficacy trials in patients with IBS-C or CC, linaclotide met all primary and
secondary endpoints encompassing abdominal and bowel symptoms.
In these trials, diarrhea was the most commonly reported
adverse event and the most commonly reported adverse event that led to study
discontinuation. Most occurrences of diarrhea were reported as mild to
moderate. More than 3,200 patients have
enrolled in one of two ongoing 18-month open label safety studies.
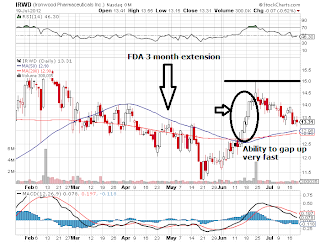
In April 2012, US FDA
notified the companies that it will require a three-month extension to complete
its review of the data supporting the New Drug Application (NDA) for
linaclotide for the treatment of irritable bowel syndrome with constipation
(IBS-C) and chronic constipation (CC).
An additional analysis of existing data was recently
requested by the FDA to further characterize the relative effect of the two
doses of linaclotide that were studied in the Phase 3 CC clinical trials.
Since this analysis
was submitted to the FDA within three months of the user fee goal date, the
date has been extended by three months, in accordance with applicable
regulation. No new data have been
requested by the agency to complete the review. FDA action is now expected by
September 2012. Ironwood and Forest continue to plan for a 2012 launch.
Ironwood is a relatively mid-cap pharmaceutical company with
market capital of $1.43B as of July 2012. It currently has revenue of $71.22M
with quarterly revenue growth of 29.70% and gross profit of $65.87M. In
addition, it has total cash of $157.65millon
which equates to about 1.47 cash per share. If the Linaclotide gets an FDA
approval, Ironwood Pharmaceutical company will get $85M milestone payment from
Forest Laboratories and $20M milestone from Almirall.
In my opinion, they have enough cash to reach their
anticipated PDUFA date on September 2012. According to their recent 2nd
quarter highlights, they also have European MAA under review which we should be
able to get a decision in the 2nd half of 2012. They are also
planning on gaining more market share by expanding global access to patient by
filing CTA (FDA of China) which is also accepted for review.
I believe that management is very confident in launching the
product commercially in 2nd half of 2012. During Digestive Disease Week
of 2012 which showcases more than 5,000 abstracts and lectures in
Gastrointestinal research, medicine and technology also had positive reviews
for the Linaclotide.
Market Need
IBS-C and Chronic
constipation (CC) are chronic conditions characterized by frequent and bothersome
abdominal and/or constipation symptoms. Patients with IBS-C experience frequent
and recurrent abdominal pain and/or discomfort and constipation symptoms, such
as infrequent bowel movements, hard/lumpy stools, and straining during
defecation.
In 2010,
approximately 40 million people in the U.S. suffered from symptoms of IBS-C or
CC, of whom an estimated 18 million patients sought medical care. Patients are
often treated with fiber and laxatives; however, according to market research,
over 70 percent are not satisfied with current treatment options. This large,
highly symptomatic, and dissatisfied patient population could benefit from new
treatments.
In my opinion, this
company should fare well in upcoming months. I definitely see an upside in stock
price from this levels. After reading the recently published randomized trial
results (http://www.nejm.org/doi/full/10.1056/NEJMoa1010863),
I feel more confident in approval of this drug. There is definitely a need for
new treatment in IBS-C and CC since current standard of therapy lacks patient satisfaction.
After close consideration and research, my price target for this company is
$16-17 in upcoming months with relative volatility due to its midcap.
Disclosure: May own
shares in this company in next 72hours.
Sources: Ironwood Pharmaceutical, New England Journal of Medicine, Yahoo Finance, Reuters, Google images





No comments:
Post a Comment